Resources
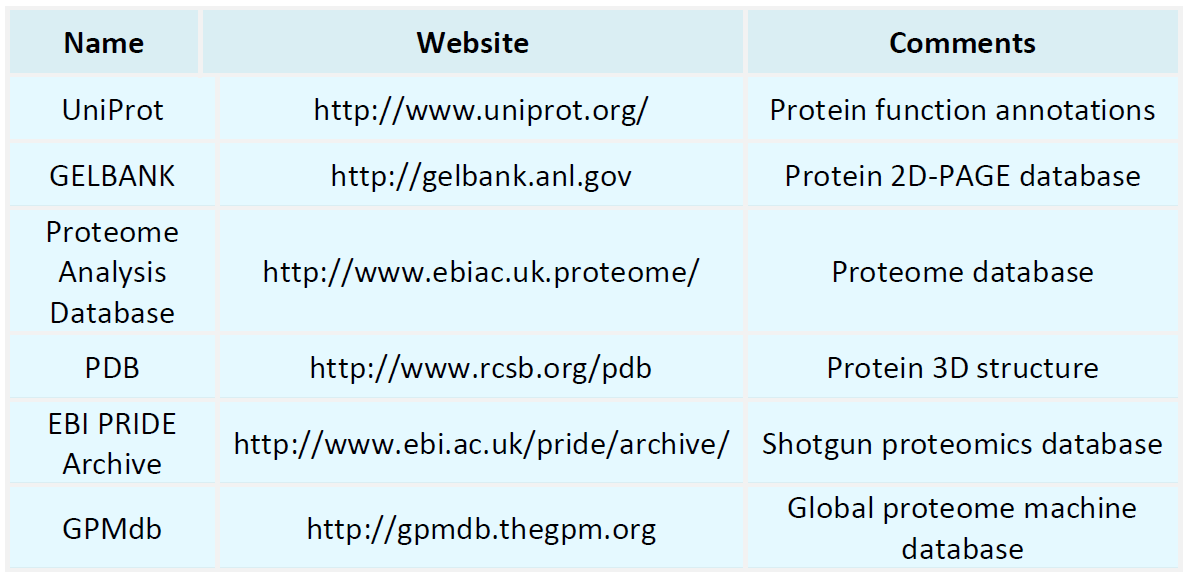
Proteomics Databases
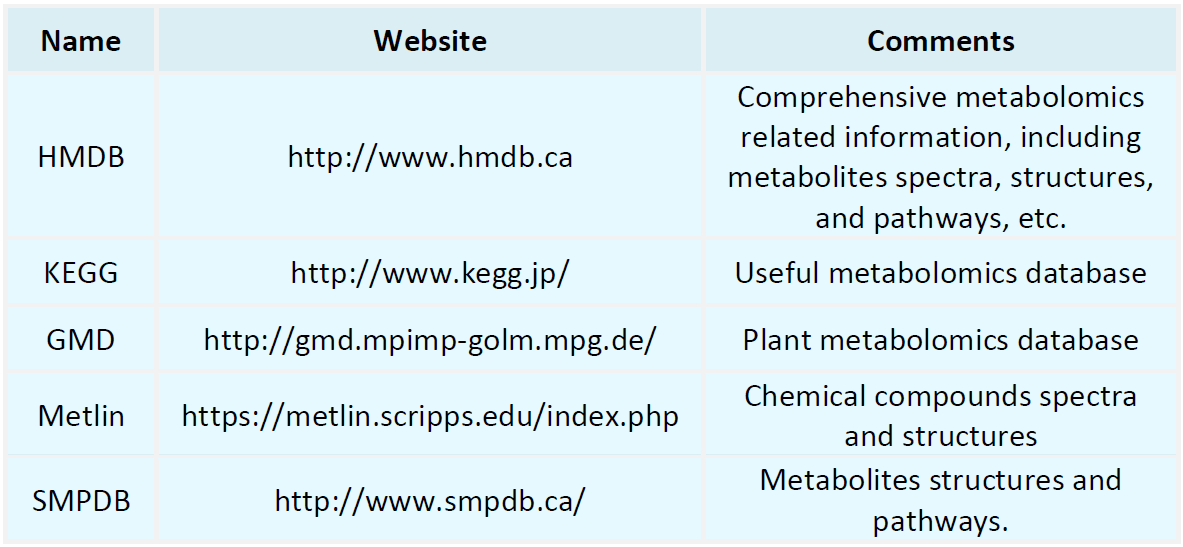
Metabolomics Databases
-
• FT-IR (Fourier Transform Infrared Spectroscopy) Analysis
FT-IR (Fourier Transform Infrared Spectroscopy) analysis is a technique widely used for the identification of chemical substances and the study of molecular structures. It is based on the specific absorption of infrared radiation by different chemical bonds and functional groups. When molecules are irradiated with infrared radiation, they absorb radiation of specific wavelengths, leading to vibrations in the chemical bonds within the molecule.
-
Protein phosphorylation is one of the most common and important post-translational modifications in cells. Phosphorylation plays a crucial role in regulating protein activity, subcellular localization, interactions, and stability by adding or removing phosphate groups on specific amino acid residues of proteins. Basic Process of Phosphorylation The phosphorylation process mainly involves two types of enzymes: protein kinases and protein phosphatases.
-
• MtoZ Biolabs: Advanced Post-Translational Modification (PTM) Proteomics Services
At MtoZ Biolabs, we offer comprehensive proteomic solutions designed to thoroughly analyze PTMs. Our state-of-the-art platform employs advanced mass spectrometry technologies coupled with highly specialized enrichment techniques, providing robust, sensitive, and precise PTM analysis. Our services cover the identification, quantification, and characterization of PTMs, offering valuable insights for academic and pharmaceutical research.
-
• MtoZ Biolabs: Comprehensive Proteomics Services
Proteomics is the large-scale study of proteins, the vital executors of life processes, which are responsible for a range of biological functions, from cellular structure to signal transduction and metabolism. Our proteomics services at MtoZ Biolabs aim to analyze and quantify the entire set of proteins expressed by a cell, tissue, or organism. We provide solutions for identifying proteins, elucidating their structure, post-translational modifications, and interactions, offering deep insights into biologica
-
• Detection of Glycosylation Modifications in Proteins
Glycosylation refers to the attachment of carbohydrate moieties (glycans) to proteins, significantly influencing their structure, stability, and function. There are two main types of glycosylation: N-linked glycosylation, where glycans are attached to the nitrogen atom of asparagine residues, and O-linked glycosylation, where glycans are attached to the oxygen atom of serine or threonine residues.
-
• Procedure for Ubiquitin Proteomics Analysis
Ubiquitin proteomics involves identifying and quantifying ubiquitinated proteins within a biological sample. This comprehensive analysis helps elucidate the dynamics of ubiquitination and its impact on cellular functions. The workflow for ubiquitin proteomics is complex and involves several critical steps, including sample preparation, ubiquitin enrichment, mass spectrometry analysis, and data interpretation.
-
• Analysis of Protein-Protein Interactions Using Pull-Down Assays
Pull-down assays are in vitro techniques used to study and validate PPIs. These assays rely on the affinity purification principle, where a bait protein is used to "pull down" a prey protein from a mixture, allowing for the identification and analysis of protein complexes. The method is versatile and can be used to confirm known interactions or discover novel ones.
-
• Workflow for Phospho Proteomics Analysis
Phospho proteomics involves the identification and quantification of phosphorylated proteins and peptides. This analysis provides insights into the dynamics of phosphorylation and its role in cellular functions. The workflow for phospho proteomics is complex and involves several critical steps, including sample preparation, protein digestion, phosphopeptide enrichment, mass spectrometry analysis, and data interpretation.
-
• Procedure for Protein Identification Using LC-MS/MS
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is a highly sophisticated and powerful technique used for protein identification and characterization. LC-MS/MS combines liquid chromatography (LC) with tandem mass spectrometry (MS/MS), allowing for the separation, detection, and identification of complex protein mixtures. LC separates peptides based on their physical and chemical properties, while MS/MS provides detailed information about the peptides' mass and structure.
-
• Procedure for Protein Mass Measurement Using MALDI-TOF
MALDI-TOF MS combines two technologies: matrix-assisted laser desorption/ionization (MALDI) and time-of-flight (TOF) mass spectrometry. This technique is known for its high sensitivity, speed, and ability to analyze large biomolecules, making it particularly suitable for protein analysis. MALDI-TOF MS is widely used in proteomics for protein identification, characterization, and quantification.
How to order?







